20 October 2023| By Abdullah Hussein
Barium sulfate (BaSO₄, barite) is one of the most challenging types of scale commonly encountered in oilfields. Due to its properties and severe impact on production, barite can become a nightmare for operators if it builds up in the production system. Generally speaking, barite is known for:
- Extremely low solubility (2.3 mg/L),
- High mechanical strength,
- Rapid formation and accumulation rates (e.g., a North Sea well dropped from 30,000 BPD to zero in 24 hours due to barite scaling),
- Tendency to accumulate radioactive materials, forming NORM scale.

Fig.1: Scale sample comprises mainly BaSO4, SrSO4 , the sample also was radioactive.
In many cases, chemical removal methods fail to dissolve barite, necessitating mechanical alternatives. Sometimes, neither approach is successful, forcing operators to cut out or replace the scaled sections of the system.
Commercial BaSO₄ dissolvers exist but face barriers like high cost (especially in large volumes), slow kinetics, and stringent conditions (e.g., high temperature).
A cost-effective alternative is polyaminocarboxylate (PAC) chelating agents (e.g., EDTA, DTPA, CDTA, NTA; see Fig. 2), which have successfully dissolved barite in oil/gas fields. PACs offer advantages:
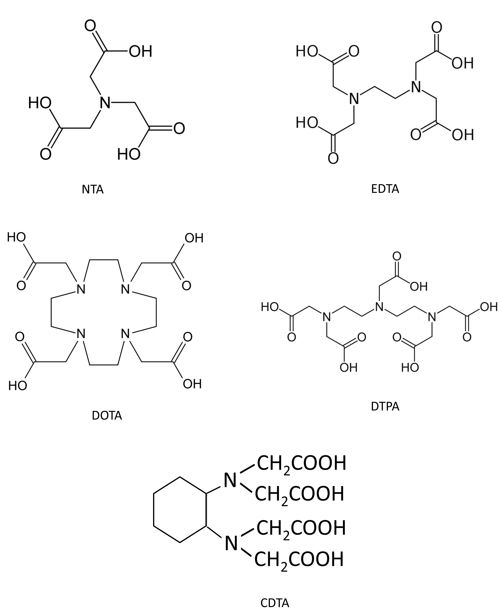
These chemicals have been used for different applications in oil and gas fields, but as scale dissolvers, they are considered :
- Low cost,
- Non-corrosive,
- Broad applicability (effective on BaSO₄, CaCO₃, CaSO₄, and some sulfide scales),
- Ease of handling with minimal safety/environmental risks,
- Flexible formulation, and tunable efficiency via catalysts.
While PAC efficiency varies in literature, their efficiency largely depends on their optimal application.
Here are some helpful tips to effectively remove barite scale using PAC at a cheap price:
- Lab Analysis:
- Determine scale composition (including polymorphs) and layer-wise distribution.
- Measure physical properties (thickness, hardness) to design cleaning.
- Dissolver Selection:
- DTPA is most effective but costly; test alternatives (e.g., EDTA may suffice at lower cost).
- Conduct lab dissolution studies (Fig. 3) to determine:
- Optimal concentration
- Catalyst use
- Temperature and soaking time
- Any side effects or risks
Fig. 3: Dissolution of barite scale sample using PACs
4. Enhance PAC performance with catalysts, such as:
- Oxalate
- Thiosulfate
- Glycolate
- Maleate
- Succinate
- Phosphonates
5. Maintain pH
- Adjust the dissolver pH > 10 (ideally 10–11.5) for best chelation performance. Note: pH above 11.5 can lead to unwanted precipitation when in contact with produced water.
6. Maintain temperature
- Adjust the temperature (> 60°C) to speed up dissolution. Downhole heat can be utilized, or external heaters/heat exchangers used for surface facilities.
7. Maintain soaking time
- from several hours to a few days depending on scale thickness. Amorphous scale generally dissolves faster.
8. Pre-Treatment:
- Flush with organic solvent to remove oil films covering barite.
- For acid-soluble components, use inhibited acid flushing to weaken scale (follow with water flush to avoid PAC-acid interference).
9. Execution:
- Soak dissolver for predetermined time (hours to days; repeat for thick/aged scale).
- Agitate/circulate if possible.
- Combine with mechanical aids (wireline tools, pigging, coiled tubing) to enhance dissolution.
--------------------------------------------------------------
Further reading
Chemical removal of barium sulfate scale